-
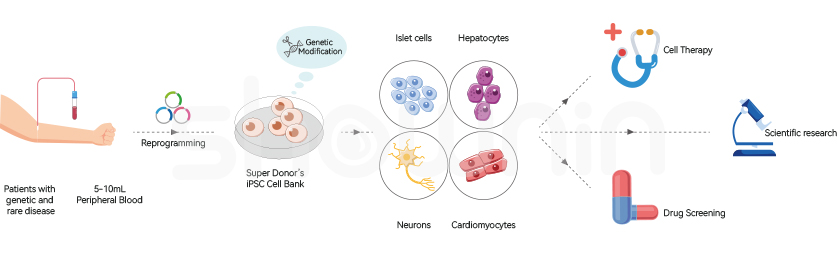
Disease Model of iPSC Cell LinesMoreIn vitro disease modeling involves generating disease-specific induced pluripotent stem cells (iPSCs) from patient somatic cells carrying disease genes. These iPSCs are then differentiated into disease-related functional cells, such as neurons, allowing the replication of the genotypic and phenotypic characteristics of the disease within a culture dish. This process enables the study of the underlying mechanisms of disease occurrence, offering novel insights for identifying effective treatment strategies and developing new therapeutic drugs.
-
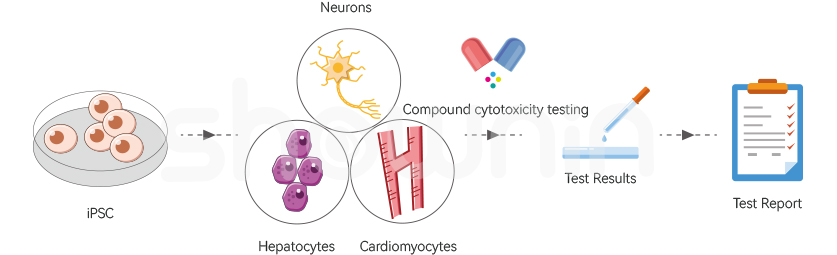
Compound Cytotoxicity TestingMoreCurrently, Shownin Biotechnologies has the capability to efficiently produce a substantial quantity of high-quality disease-specific and normal population induced pluripotent stem cells (iPSCs) in vitro. This enables us to offer researchers an unlimited and consistent supply of iPSCs for simulating clinical phase I trials and conducting in vitro toxicity tests for drug evaluation. This approach serves as a partial alternative to the conventional clinical phase I trials conducted directly on human subjects, mitigating the potential harm from drug toxicity during the clinical phase I stage and significantly bolstering the protection of subjects' rights.
-
Compound Cellular Efficacy TestingMoreShownin Biotechnologies has now achieved the capability to produce a substantial quantity of disease-specific and normal induced pluripotent stem cells (hiPSC) with consistent quality. This includes iPSC cell lines for diseases such as Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), among others. Researchers can leverage these disease-specific iPSC cell lines to derive functional cells for effective testing of disease-related drug efficacy, facilitating the identification of candidate drugs and their effective dosage ranges in the treatment of various diseases.

